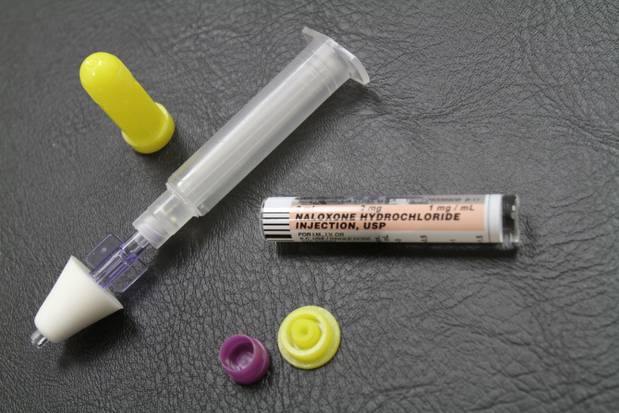
 The FDA recently approved the use of Naloxone as a prescription treatment in the household for use in the case of an opioid overdose, in the form of an intramuscular auto-injector – similar to EpiPens (epinephrine) carried by those with severe allergies.
The FDA recently approved the use of Naloxone as a prescription treatment in the household for use in the case of an opioid overdose, in the form of an intramuscular auto-injector – similar to EpiPens (epinephrine) carried by those with severe allergies.
Naloxone is known to immediately reverse the effects of opioid overdoses and has been instrumental in saving the lives of many in the case of accidental overdose either through the illicit or prescription use of opioid drugs.
It has previously seen wide support across the US, with the American Medical Association and the American Public Health Association having carried policies supporting its widespread use since 2012 – and therefore in many states Naloxone has been available to opioid users for a number of years. However, full FDA approval ensures that the drug is available to all in need accross the US, and the approval of an intramuscular form of the treatment maximises potential effectiveness.
Read more on the FDA website here, and read more about opioid overdoses and what you can do in such a case here.
